Boiler Combustion Analysis 101
By: Roy Collver
 Roy’s Background and Upcoming Class
Roy’s Background and Upcoming Class
Roy Collver teaches an advanced 5-week class on Mastering Condensing Boiler Design in Hydronic Systems with the folks at HeatSpring. If you need to increase your skills and confidence around selling, quoting, designing, setting up controls, or troubling shooting Condensing Boilers in new construction or retrofit applications, this class is for you. Each class is capped at 50 students, but there are 30 discounted seats. Get your discount and sign up for Mastering Condensing Boilers in Hydronic Systems.
Here’s what John Siegenthaler, author or “Modern Hydronic Heating” says about Roy’s work, “When I have a detailed question about the inner operation of a modulating / condensing boiler, Roy Collver is the first person I contact. The investment in Roy’s HeatSpring course is a fraction of the cost of a single mod/con boiler, but it will teach you concepts, procedures, and details that will return that investment many times over.”
Enter Roy —
I was reading a boiler installation manual lately. It stated the following in the “Start-up” section: “Using an appropriate Oxygen or Carbon Dioxide analyzer, take a sample of the flue gas”. The manual then goes on to define that the sample must fall within a very finite range for carbon dioxide.
This really worries me.
I spent many years doing combustion tests on a very wide variety of burner systems that ranged all the way from atmospheric coal-to-natural gas conversion burners in gravity “octopus” residential central air furnaces – to fan assist, nozzle-mix power burners installed in large and small hot water and steam boilers – to pre-mix industrial burners of all makes and flavors from low (70°F) to high (3600°F) temperature applications.
After many, many years of almost daily operation of sophisticated combustion analyzers in a wide variety of applications, I considered myself no more than an intermediate level practitioner of the fine Art of combustion analysis. You can spend your entire life doing this and not master it.
I have avoided writing about this topic for many years, because I thought it a far too complex a subject for a short article. However when I see that manufacturers are making statements like the one above – I guess I figure it’s time to tackle it. We are looking at a serious safety issue here – and it will take me a couple of issues to cover the topic in detail. At the end of the day, all I can really suggest is that you contact a manufacturer of combustion analyzer equipment, and take as much training as you can from them. By the way – if you are NOT a trades-person, trained and licensed as a gas-fitter, do NOT think this article will qualify you to mess with this stuff. Put it down, and call someone who is qualified.
Some important questions follow:
• What is a combustion analyzer?
• What does it analyze?
• How does it work?
• Where do you get one?
• How do you know if it is a good one?
• How do you use it?
• What can happen if you screw up?
• Where do you learn how to use one?
• When do you have to use one?
To even start to answer these questions, we have to go back to our high school chemistry class and look at the basic combustion process.
Mix a fuel (a bunch of chemicals) with oxygen – provide an ignition source – and you will get a combustion process occurring. The combustion process will produce heat, and a bunch of different (unwanted) chemicals. A boiler is simply a device designed to burn a fuel and then transfer the heat from the combustion process into the water that will heat the space. Inherent in this process is the transfer of any other products of combustion (the bunch of unwanted chemicals) to somewhere will it won’t hurt anyone. All a combustion analyzer does is sample the unwanted chemicals and tell you how complete the combustion process was.
Let’s look at the process of combining natural gas and air, and then lighting it up in a boiler to produce heat. The same process works with all fuels, but the components are different. I am using natural gas as an example because it is by far the most common fuel used in boilers in North America, and the methane molecule is quite simple so that the molecular bonding that takes place is easy to visualize. Before I start getting all kinds of letters from you chemists out there, I freely admit that I am greatly simplifying the process and the chemical components that are involved. In our heating industry, a basic understanding of the process is more important to communicate than all of the scientific minutiae. If you want to get into the details of covalent bonds, electron bonding pairs, electron shells, etc., I recommend you enroll in a chemical engineering course.
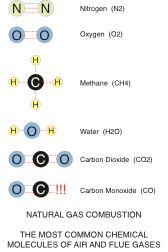
 Here are the molecules we are going to be talking about:
Here are the molecules we are going to be talking about:
The basic molecule of natural gas is methane. Methane has a single carbon atom, which has four hydrogen atoms bonded to it. Air however, is not pure oxygen. It is normally made up of 21% Oxygen and 78% Nitrogen with a bunch of other junk making up the remaining 1%.
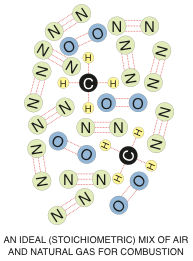
When you mix one methane molecule (CH4) with an oxygen molecule (O2) and then add enough heat (around 1,100°F – 590°C) – you get ignition.
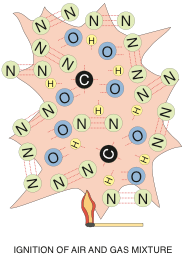 Ignition results in very fast oxidization of the methane molecule – wherein the chemical bonds of both molecules are broken and then re-formed. In a perfect mix of gas and air, the methane and oxygen will form water vapour and carbon dioxide, and release a whole bunch of heat (1,000 Btuh for every cubic foot of natural gas). You can see from the illustration below, that the nitrogen is just along for the ride. It gets heated up by the process, but does not normally form any new chemical bonds unless there is extra (excess) air and the flame is very hot. Sometimes you can get some really nasty NOx forming (NO, NO2), but modern burner technology has gone a long way towards reducing this reaction and we won’t be discussing it in this article.
Ignition results in very fast oxidization of the methane molecule – wherein the chemical bonds of both molecules are broken and then re-formed. In a perfect mix of gas and air, the methane and oxygen will form water vapour and carbon dioxide, and release a whole bunch of heat (1,000 Btuh for every cubic foot of natural gas). You can see from the illustration below, that the nitrogen is just along for the ride. It gets heated up by the process, but does not normally form any new chemical bonds unless there is extra (excess) air and the flame is very hot. Sometimes you can get some really nasty NOx forming (NO, NO2), but modern burner technology has gone a long way towards reducing this reaction and we won’t be discussing it in this article.
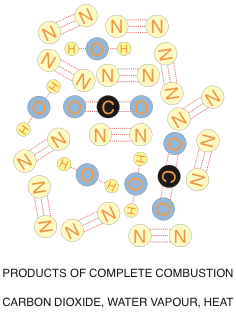
Let’s cut to the chase and get to the reason why we want to analyze our combustion flue gases in the first place.
What happens if you have less than the ideal gas/air mixture?
Not enough air will result in a potentially dangerous situation. If we are short of oxygen, or we don’t complete the combustion process for other reasons such as flame impingement, some of the carbon atoms will only grab onto a single oxygen atom, and you will be left with a carbon monoxide molecule. In severe cases, if no oxygen atoms grab onto the carbon atom, you will end up with plain old carbon soot.
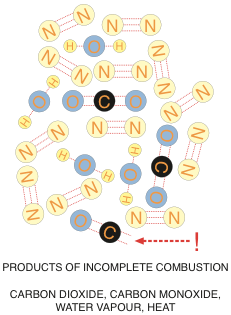
Carbon monoxide is an unstable molecule with two molecular bonds available – searching for something to bond to. If it can’t find an oxygen atom – well then a red blood cell will do just fine. Once carbon monoxide hooks up with your red blood cells, it makes it impossible for the red blood cells to do their job and pick up oxygen on their way through your lungs. If enough red blood cells pick up carbon monoxide molecules – you die. Repeat – you die.
That is why you really, really want to know what you are doing when you pull out your combustion analyzer. You want to know for sure that it works properly – so you had better be prepared to have it checked and calibrated on a regular basis. The manufacturer of your machine will tell you how often this should be done. Don’t skimp on this – someone’s life could depend on it. Whenever and wherever I use my analyzer, I pretend that the appliance I am testing is going to be next to my bedroom. Would I feel comfortable sleeping next to the boiler after I am done? This is a good visualization exercise for anyone doing this kind of work.
Do you know what you are doing? Have you been trained in the use of the particular analyzer you are going to use? Are you like the fellow below who doesn’t look like he is quite sure what buttons to push or where to stick the little probe thingie?
Next issue, we will examine a variety of types of combustion analyzers and give you some valuable tips on how to use them. Most of you will not need one as fancy as the one I have (pictured above), but what features are really required?
The main reason we test appliance flue gasses, is to optimize the combustion efficiency of the burner system, not to specifically check for carbon monoxide. The analyzer looks at the chemical composition of the flue gasses and their temperature, and then calculates how much heat is being recovered from the combustion process. With even the best pre-mix burners available today, it is impossible to achieve perfect combustion without introducing some excess air. In order to maximize efficiency, we want to reduce the excess air as much as possible without getting to the point where we are producing excessive carbon monoxide.
The diagram below, shows that we heat up much more nitrogen and oxygen than we need to in order to not have too much carbon monoxide. If we don’t recover that heat, it’s wasted when it’s vented to atmosphere.

Modern condensing Boilers absolutely require complete combustion in order to achieve their rated efficiencies, but the cooler they operate (deeper condensation), the less heat the excess air has. The strategy is to run the boiler as cool as possible, and at the same time, cut back on the excess air as much as possible without getting into a situation where you start to produce carbon monoxide. This is a very fine line, and one you never want to cross. That is why an accurate, properly calibrated combustion analyzer AND the knowledge and experience required to operate it properly is an absolute must, if you want to sleep well at night.
Sleep tight!
Roy Collver




Join the conversation: