The importance of neutralizing condensate produced by high efficiency gas fired appliances
Most new residential and commercial heating and hot water systems include a furnace, boiler or domestic water heater of the high efficiency condensing type. Condensing appliances extract additional BTUs from the water vapor in the flue gas. When the flue gas drops below its dew point of approximately 135 degrees Fahrenheit, it starts to condense and the resulting liquid is acidic and requires treatment to avoid damage to piping systems, sewerage systems, treatment facilities, septic systems and other items it may come in contact with.
A general rule of thumb, one gallon of condensate is produced for every 100,000 BTUs of input providing the appliance is operating in full condensing mode. If an appliance having 100,000 BTUs of input operates for 1200 hours annually, it will produce approximately 1200 gallons of condensate.
The acidic level of the condensate is measured as its pH. Most condensate from natural gas appliances will have a pH of between 2.9 and 4 with 7 being neutral. The actual pH will vary according to the actual chemical makeup of the fuel that is being burned. Condensate contains different types of acids that are corrosive to many materials. The products of natural gas combustion generally include nitrogen oxides, sulfur oxides and hydrogen chloride, as well as water vapor and carbon dioxide. Hydrogen chloride is a result of the combustion of chlorides. These chlorides (salts) are contained in the combustion air.
Taking combustion air from outside generally reduces the amount of chlorides. Nitrogen oxides are a normal by product of combustion. Sulfur compounds are added to the natural gas in small amounts as an odorant.
Condensation of flue gas produces an acidic solution containing concentrations of nitric, nitrous, sulfuric, and sulfurous and hydrochloric acids. These acids can become more concentrated by repeated condensing and evaporation on heat exchangers and flues. Some say condensate from natural gas appliances is no more acidic than vinegar, urine and some citrus juices. Although the pH may be similar, it is a different type of acid and you can be certain no one is dumping thousands of gallons of these liquids into their drains.
A pH of 4 can damage drainage pipes, septic tanks, treatment plants and other materials handling waste water. The pH scale is not linear. Each whole number step below 7 is 10 times more acidic than the next higher number.
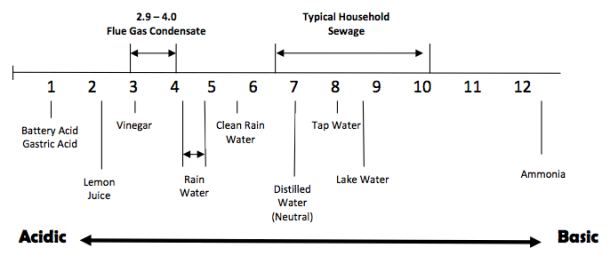
Treated condensate should be as close to neutral as possible with 5 being the minimum. Most national and state codes 1 prohibit anyone from allowing acidic liquid into a drainage system without treating it to raise its pH.
Treatment of this condensate is accomplished by exposing the liquid to a media containing calcium carbonate. Calcium carbonate can be found in rocks, egg shells and shells of marine animals. The most common material used is limestone or a limestone product.
There are two important factors when considering condensate neutralizers – the percentage of calcium carbonate and the amount of surface area of the media.
Look for products containing:
- A minimum of 90% calcium carbonate
- Media with more surface area produces more effective results
The acid is converted to water, carbon dioxide and mineral salts when exposed to the calcium carbonate media. The carbon dioxide will generally stay diluted in the water and pass out of the neutralizer. The mineral salts generally settle at the bottom of the neutralizer. Over time the surface of the media becomes coated or clogged and the efficiency is reduced. The media should be replaced at this point.
How do you know if the neutralizer is working? Manufacturers of condensate neutralizers provide sizing guide lines and recommended maintenance (media replacement) intervals. The application should be monitored and checked periodically for optimal results. Sizing and maintenance intervals should be adjusted accordingly.
It is the responsibility of the plumbing professional to follow state and local plumbing codes including proper disposal of condensate and equally important for the end user to provide ongoing maintenance ensuring proper function of the unit. An example of damage caused by improperly treated condensate can be seen in the picture at the beginning of this paper. Damage can occur underground and in unseen, remote locations. Imagine digging up your basement floor to replace a rotted out metallic drain line!
¹ National Standard Plumbing Code Chapter 6; Massachusetts Plumbing Code CMR248 Chapter 10
Mike Bernasconi is VP and co-owner of Neutrasafe Corporation, a Massachusetts based manufacturer of condensate neutralizers. Bernasconi, a licensed Master Plumber and hydronics specialist, has been working in the HVAC industry for 35+ years and is well known in the industry for hydronics design and execution. With the onset of condensing boilers, furnaces and hot water heaters, the installation of a condensate neutralizer is now a mandatory requirement. Bernasconi designed a condensate neutralizer with the installer in mind. His product contains a clear body, clean, consistent sized high quality media and offers ease of servicing. Neutrasafe Corporation partners with manufacturer representatives throughout the U.S. to distribute its products. Products can be viewed at www.neutrasafe.com. He can be reached at mike@neutrasafe.com





Join the conversation: